Question Code: 044/2022 (A)
Name of Post: Archaeological Chemist
Department: Archaeology
Cat. No: 131/2020
Date of Test: 26.04.2022
1. The residual molar entropy of crystalline CO at absolute zero is:
(A) R ln2
(B) 2 − R ln
(C) 4 R ln
(D) 4 − R ln
2. The rate constants of a reaction at 100K and 200K are 0.02 −1 s and 1 0.2 − s . What will be the value of activation energy, Ea of this reaction?
image
Correct Answer : C
3. Using molecular orbital theory the bond order of 2− O ion is found to be :
image
Correct Answer : C
Question deleted
4. Which among the following is not an example for liquid crystal?
(A) Ethyl p-Azoxy benzoate
(B) Ethyl benzoate
(C) Cholesteryl benzoate
(D) p-Azoxy phenetole
5. For a particle in one dimensional box, the difference in energy between energy levels 3 n =and 2 n = was found to be 15 units. What will be the difference in energy for the levels 2 n =and 1 n = for this system :
(A) 6
(B) 9
(C) 0
(D) 15
6. Zinc oxide exists as a white coloured substance at room temperature. On heating it turns yellow due to:
(A) Frenkel defect
(B) Schottky defect
(C) Metal excess defect
(D) Metal deficiency defect
7. A character table of C point group contains a one dimensional representation which is symmetric to the principal rotation axis and antisymmetric with respect to the centre of inversion, i. What Mullikan symbol should be assigned to the representation?
(A) Bu
(B) Ag
(C) Bg
(D) Au
8. Which among the following is not a molecular mechanics force field?
(A) AMBER
(B) CHARMM
(C) GROMOS
(D) PM3
9. Select the correct statement regarding Langmuir adsorption isotherm :
(A) Adsorption takes place in multilayer
(B) Adsorption sites are equivalent and the surface is uniform
(C) Adsorbed molecules interact with each other
(D) None of these
10. The normalized wave functions ψ1 and ψ 2 of the sp hybrid orbitals formed by the combination of one 2s and one 2p orbital are:
Correct Answer : A
11. On mixing dilute AgNO3 solution with dilute NaI solution, a negatively charged colloidal sol of AgI was obtained. Select the appropriate condition for the formation of this sol :
(A) Addition of AgNO3 to slight excess of NaI
(B) Addition of NaI to slight excess of AgNO3
(C) Mixing equal amounts of AgNO3 and NaI
(D) None of these
12. The entropy change involved when volume of 1 mol of any perfect gas is tripled at constant temperature is :
(A) 2.303Rlog 3
(B) R log1/ 3
(C) 2.303R log1/ 3
(D) 2.303R log 3 /2
13. The rate of an enzyme catalysed reaction [E] + [S] → [ES] is obtained by Michaelis-Menten equation. If the concentration of the substrate [S] is very small when compared to the Michaelis constant KM, the rate of the reaction will be :
(A) First order with respect to [S]
(B) Zero order with respect to [ S ]
(C) First order with respect to total enzyme concentration [E0]
(D) Both (A) and (C)
14. Using Debye Huckel Onsagar equation deduce the factor responsible for increasing the conductance ratio of a strong electrolyte :
(A) decreasing concentration
(B) increasing temperature
(C) small dielectric constant of the solvent
(D) increasing valence of the ions
15. ESR spectra of benzene radical anion gives :
(A) doublet lines
(B) quartet lines
(C) septet lines
(D) triplet lines
16. The number of degrees of freedom at the eutectic point of a two component system is :
(A) 1
(B) 0
(C) 2
(D) 3
17. The source of error encountered in ab-initio calculations include :
(A) Born – Oppenheimer approximation
(B) Omission of relativistic effects
(C) Use of incomplete basis set
(D) All of the above
18. Among the following symmetry elements, the element absent in trans-dichloroethylene is :
(A) C2 proper rotaion axis
(B) vertical plane of symmetry, σ v
(C) horizontal plane of symmetry, σ h
(D) inversion centre, i
19. The rotational spectrum of rigid diatomic molecule consists of lines which are equally spaced.
If B is the rotational constant the spacing between the lines is equal to:
(A) 2B
(B) B
(C) 4B
(D) 3B
20. The angular momentum operator, Lxˆ is:
Correct Answer : A
21. The factor which affects the diffusion current in polarography is :
(A) number of electrons involved in electrode reaction
(B) diffusion coefficient of elecroactive species
(C) bulk concentration of the electroactive material
(D) all of these
22. The reducible representation of the hybrid orbitals of a molecule belonging to C2v point group was found to be 2A1 + B1 + B2 . From the character table given below, predict the hybridization of the molecule :
Correct Answer : C
23. The following pair of compounds are :
(A) Geometrical isomers
(B) Diastereomers
(C) Enantiomers
(D) Meso compounds
24. Which of the following is the most reactive towards substitution by SN1 mechanism?
Correct Answer : C
25. Which of the options shows the same compound as the following?
Correct Answer : A
26. Which of the following statements is not correct for alkyl halide?
(A) Order of reactivity of alkyl halides towards E2 dehydrohalogenation is found to be 3° > 2° > 1°
(B) As branching at carbon increases, E1 mechanism is favored as compared to SN1 mechanism
(C) In E2 elimination different stereoisomer (diastereomer) converts into different stereo product
(D) In most unimolecular reactions of alkyl halide E1 reaction is favored over SN1 reaction
27. Which of the following is most acidic?
Correct Answer : B
28. Which of the following is the intermediate in Wolff’s reaction?
(A) Ketene
(B) Carbene
(C) Carbocation
(D) Free radical
29. Which among the options can function as ‘X’ in the following reaction?
(A) Conc. H2SO4
(B) PCl 5
(C) SOCl
(D) Any of the above 2
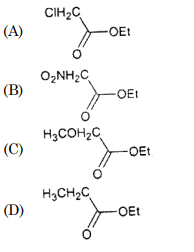
30. Which of the following ester is most reactive towards alkaline hydrolysis?
Correct Answer : B
31. Which among the following is most basic?
(A) C6H5NH2
(B) C 6H5CH2NH2
(C) p − NO2C6H4NH2
(D) m − NO2 − C6H4NH2
32. Which is the highest energy conformation of cyclohexane?
(A) Chair
(B) Twist boat
(C) Half-chair
(D) Boat
33. Which among the following is not aromatic?
Correct Answer : C
34. Which of the following will be correct if a reaction going in 80% enantiomeric excess?
(A) The product contains 80% R enantiomer and 20% S enantiomer
(B) The product contains 50% R enantiomer and 30% S enantiomer
(C) The product contains 90% R enantiomer and 10% S enantiomer
(D) None of the above
35. Choose the major product of the following reaction :
Correct Answer : B
36. Which of the following is an electrocyclic reaction?
Correct Answer : A
37. HOMO for hexa-1,3,5-triene under photochemical condition is :
(A) ψ 5
(B) ψ 2
(C) ψ 4
(D) ψ 3
38. Which of the following is the application of ion exchange chromatography?
(A) The softening of hard water
(B) The demineralisation of water
(C) Both (A) and (B)
(D) None of the above
39. Which is the correct order of increasing wave number of the stretching vibrations of
(1) C-H (alkane), (2) C-H (alkene), (3) C-H (alkyne), and (4) C-H (Arene)?
Correct Answer : A
40. Which among the following shows carbonyl stretching frequency at 1715 −1 cm ?
41. Nuclei having both atomic number and mass number even have ____________ spin.
(A) Integral spin
(B) Half integral spin
(C) Zero spin
(D) Positive spin
42. Which of the following has three types of hydrogens in the following compounds?
Correct Answer : D
43. Identify the reaction :
(A) Gabriel phthalimide synthesis
(B) Chan-Lam coupling
(C) Buchwald-Hartwig Reaction
(D) Ullmann reaction
44. Fmoc is :
(A) 6-fluorenylmethoxycarbonyl
(B) 7-fluorenylmethoxycarbonyl
(C) 9-fluorenylmethoxycarbonyl
(D) 7-fluorenylethoxycarbonyl
45. The precursor of lanosterol is :
(A) Homoserine
(B) Asparagines
(C) Squalene
(D) Tryptophan
46. In maltose, the glucose units are connected through :
(A) β(1 → 4 ) glycosidic bond
(B) α(1 → 4 ) glycosidic bond
(C) α,β( 1 → 4 ) glycosidic bond
(D) α,β( 1 → 2 ) glycosidic bond
47. Which of the following is not an organometallic compound?
Correct Answer : C
48. Zerevitinov method is used to estimate the number of which group in organic compound?
(A) − NH2
(B) − OH
(C) SH −
(D) All of these
49. Grignard reagent does not react with the following functional group :
(A) O > C =
(B) > C = C <
(C) OH −
(D) X−
50. Organo zinc compounds are involved in the reaction :
(A) Reformatsky reaction
(B) Gattermann reaction
(C) Friedel-Crafts reaction
(D) Knovenagel reaction
51. Which is the organometallic compound capable of adding on to a Carbon-Carbon Double bond is :
(A) R ZnX
(B) RzZn
(C) R Li
(D)R MgX
52. Which of the following does not have an α, unsaturated Carbonyl group? β
(A) Androsterone
(B) Testosterone
(C) Progesterone
(D) Cortisone
53. Which of the following acts as a quenching gas in Geiger Muller Counter?
(A) Argon gas
(B) Krypton gas
(C) Hydrogen gas
(D) Alcohol
54. Which of the following material is used as the insulation between inner and outer electrodes of the ion Chamber?
(A) Poly acryl amide
(B) Polytetrafluoroethylene
(C) Ceramic
(D) Plastic
55. Liquid samples must be counted using ionisation Chamber by placing them in which of the following?
(A) Test tube
(B) Cuvette
(C) Ampoules
(D) Faraday Cup
56. Neutron activation analysis is used to estimate the amount of an element in samples of :
(A) Jewels
(B) Precious stones
(C) Ancient Coins
(D) All of these
57. Which of the following acting as an Usanovich acid?
Correct Answer : D
58. ____________ is an amphoteric solvent.
(A) C2H5OH
(B) C6H5N
(C) HF
(D) Liquid NH 3
Question deleted
59. Which Physical property is not used in thermal methods of analysis of the samples?
(A) Mass
(B) Temperature
(C) Volume
(D) Pressure
Correct Answer : B
61. Assume the accepted value to be 1.45, which of the following options correctly describes the students experimental data? Trial 1measurement 1.29, Trial 2 measurement 1.93, Trial 3 measurement 0.88 :
(A) Accurate but not precise
(B) Precise but not accurate
(C) Both accurate and precise
(D) Neither accurate nor precise
62. In Mohr’s method, if acidic solution is used :
(A) Chromate ions are decreased
(B) Chromate ions are increased
(C) Both (A) and (B)
(D) None of the above
63. Which one of the following do not have a permanent dipole?
Correct Answer : C
64. Which is the reason for ( Fe2CO9 ) being diamagnetic?
(A) Presence of the CO as bridge group
(B) Presence of Monodentate ligand
(C) Metal-Metal (Fe − Fe ) bond in molecule
(D) Resonance hybridisation of CO
65. Geometrical isomerism is exhibited by :
Correct Answer : A
66. The degree of ____________ between the metal ion and the ligand directly influences the stability of the complex in solution.
(A) Association
(B) Dissociation
(C) Ionisation
(D) Freedom
67. Actinides have higher tendancy to form complexes than lanthanides, due to :
(A) Smaller charge and larger size
(B) Higher charge and smaller size
(C) They are radioactive
(D) They are electropositive
68. ____________ is a Z diamensional nanomaterial.
(A) Quantum dot
(B) Quantum wire
(C) Quantum well
(D) Carbon nanotube
Question deleted
69. Reaction with CN CH3 with I CH3Mg excess will finally result in the formation of :
(A) CH3 CHO
(B) C2H5CHO
(C) CH CO CH3
(D) ( CH3 ) 3 COH
70. Which of the following is an example of top down approach of synthesis of the nanomaterials?
(A) Physical Vapour Deposition
(B) Chemical Vapour Deposition
(C) Sputtering
(D) None of these
71. Nanosized polymer built from branched units are called :
(A) Dendrimers
(B) Composites
(C) Carbon based materials
(D) Metal based materials
72. Regarding nanomaterials, consider the following statements :
(1) They have reduced imperfection compared to the bulk material
(2) They are used in fuel cells
(3) They have high surface energy
(4) They have a large fraction of surface atoms
Out of these statements
(A) (1), (2), (3), (4) are correct
(B) (1), (2), (3) are correct
(C) (1), (2), (4) are correct
(D) (2), (3), (4) are correct
73. Nanoscale titanium Oxide increases the :
(A) Resistance
(B) Stability
(C) Conductivity
(D) Ductility
Question deleted
74. The green synthesis methods should have :
(A) Low atom efficiency
(B) High efficiency
(C) High energy requirements
(D) High harmful products
75. In green Chemistry, we must use feed stock derived from annually renewable resources or from :
(A) Plants
(B) Chemicals
(C) Organic Compounds
(D) Abundant wastes
76. An ideal solvent facilitates the :
(A) Dissolving property
(B) Mass transfer
(C) Titration
(D) Combustion
77. IUPAC name of repeating unit of crown ethers is :
(A) Ethyleneoxy
(B) Dimethyl eneoxy
(C) Ethoxy
(D) Diethylether
78. Which of the following statements is not true about Cyclodextrins?
(A) They are polysaccharides
(B) They have a hydrophilic outer surface
(C) They are useful for formulating hydrophilic APIs
(D) They are useful for formulating hydrophobic APIs
79. ____________ are macropolycyclic poly aza – poly ethers.
(A) Cyclophanes
(B) Cryptands
(C) Rotaxanes
(D) Crown ethers
80. A Calixarene is a cyclic oligomer based on a methylene-linked :
(A) Acids
(B) Phenols
(C) Aldehyde
(D) Ketones
81. Read the following literary works in Malayalam. Select the book not written by Lalithambika Antharjanam :
(A) Agnisakshi
(B) Athma Kadhakkoru Aamugham
(C) Ambalamani
(D) Seethamuthal Sathyavathi Vare
82. Choose the correct pairs :
1. Kumaran Asan (a) Nair Service Society
2. K. Kelappan (b) Sree Narayana Dharma Paripalana Yogam
3. Swami Vagbhatananda (c) Sudharma Suryodaya Sabha
4. Pandit K.P. Karuppan (d) Athma Vidhya Sangam
(A) 1-(d), 2-(b), 3-(c), 4-(a)
(B) 1-(a), 2-(c), 3-(b), 4-(d)
(C) 1-(b), 2-(a), 3-(d), 4-(c)
(D) 1-(a), 2-(d), 3-(c), 4-(b)
83. Choose the correctly matched Biography :
(A) Jeevitha Samaram – V.T. Bhattathirippadu
(B) Kazhinja Kalam – C. Kesavan
(C) Kannirum Kinavum – K.P. Kesavamenon
(D) Ormmayude Olangalil – G. Sankara Kuruppu
84. Who among the following is not a winner of Jyan Preed Puraskar?
(A) Vaikom Muhammed Basheer
(B) Thakazhi Siva Sankara Pillai
(C) S.K. Pottakadu
(D) G. Sankara Kuruppu
85. Choose leader associated with Ezhava Memorial?
(A) K. Kelappan
(B) Dr. Palpu
(C) Chattambi Swamikal
(D) T.K. Madhavan
86. Assertion : The Election and Electoral College for the President of India is same as that of the Vice President of India.
Reason (R) : Vice President of India is the highest dignitary of India next after the President.
(A) Both assertion and reason are true and R is the correct explanation of Assertion
(B) Both assertion and reason are true but the reason given is not the correct explanation of Assertion
(C) Assertion is true but reason is false
(D) Assertion is false but reason is true
87. Assertion : In a parliamentary democracy the President cannot function without the aid and advice of the cabinet Headed by the Prime Minister.
Reason (R) : Parliamentary system of government represents the whole Nation.
(A) Both assertion and reason are true and R is the correct explanation of Assertion
(B) Both assertion and reason are true but the reason given is not the correct explanation of Assertion
(C) Assertion is true but reason is false
(D) Assertion is false but reason is true
88. Assertion : ‘Preventive Detention’ is included in the chapter on Fundamental Rights mentioned in the Indian Constitution.
Reason (R) : Preventive Detention without trial can subsist only as long as the legislature permits.
(A) Both assertion and reason are true and R is the correct explanation of Assertion
(B) Both assertion and reason are true but the reason given is not the correct explanation of Assertion
(C) Assertion is true but reason is false
(D) Assertion is false but reason is true
89. Assertion : The people of India enjoy the Fundamental Rights of Equality, Liberty Religion and Right to Education and Culture.
Reason (R) : The Judiciary cannot enforce the state to implement fundamental rights.
(A) Both assertion and reason are true and R is the correct explanation of Assertion
(B) Both assertion and reason are true but the reason given is not the correct explanation of Assertion
(C) Assertion is true but reason is false
(D) Assertion is false but reason is true
90. Assertion : The normal tenure of Lok Sabha is 5 years but it may be dissolved earlier by the president of India.
Reason (R) : The Parliament shall meet at least once a year and the interval between two consecutive sessions shall be 5 months.
(A) Both assertion and reason are true and R is the correct explanation of Assertion
(B) Both assertion and reason are true but the reason given is not the correct explanation of Assertion
(C) Assertion is true but reason is false
(D) Assertion is false but reason is true
91. Assertion : Article 312 of the constitution provides that any proposal to create an All India Service should finanate from the Council of States ( ( ( Rajya Sabha)
Reason (R) : Rajya Sabha is the upper chamber of the Parliament.
(A) Both assertion and reason are true and R is the correct explanation of Assertion
(B) Both assertion and reason are true but the reason given is not the correct explanation of Assertion
(C) Assertion is true but reason is false
(D) Assertion is false but reason is true
92. Assertion : No minimum age is prescribed for the appointment as a judge of the supremecourt.
Reason (R) : The original jurisdiction of the supreme court is mentioned in article 149 of the Constitution.
(A) Both assertion and reason are true and R is the correct explanation of Assertion
(B) Both assertion and reason are true but the reason given is not the correct explanation of Assertion
(C) Assertion is true but reason is false
(D) Assertion is false but reason is true
93. Assertion : The President of India can declare National Emergency under article 352 of the Indian Constitution due to war or external aggression or armed rebellion.
Reason (R) : National Emergency is imposed in India in 1962, 1971, 1975 and in 1999.
(A) Both assertion and reason are true and R is the correct explanation of Assertion
(B) Both assertion and reason are true but the reason given is not the correct explanation of Assertion
(C) Assertion is true but reason is false
(D) Assertion is false but reason is true
94. Assertion : The Governor consults High Court and Public Service Commission before posting District judges in the judicial service of the state.
Reason (R) : Direct control over district courts are vested in the supreme court.
(A) Both assertion and reason are true and R is the correct explanation of Assertion
(B) Both assertion and reason are true but the reason given is not the correct explanation of Assertion
(C) Assertion is true but reason is false
(D) Assertion is false but reason is true
95. Assertion : Amendment procedure of Indian constitution is mentioned in Article 362 of the constitution.
Reason (R) : There are three methods for the Amendment of the Indian Constitution.
(A) Both assertion and reason are true and R is the correct explanation of Assertion
(B) Both assertion and reason are true but the reason given is not the correct explanation of Assertion
(C) Assertion is true but reason is false
(D) Assertion is false but reason is true
96. Assertion : The Right to Information Bill was passed by the Parliament of India on 15th June 2005 and came into force with effect from 12th October 2005.
Reason (R) : Offices of the Governors and Chief Ministers of states are not legally obliged
under the RTI Act.
(A) Both assertion and reason are true and R is the correct explanation of Assertion
(B) Both assertion and reason are true but the reason given is not the correct explanation of Assertion
(C) Assertion is true but reason is false
(D) Assertion is false but reason is true
97. Which of the following statement about the Fundamental Duties mentioned in the Indian constitute is not correct?
(A) Fundamental Duties are recommended by the Swaran Singh committee
(B) Fundamental duties are mentioned in the part 4 of the constitution
(C) Fundamental duties can be enforced through the writ jurisdiction under Article 32 of the constitution
(D) Fundamental duties are incorporated in the Indian Constitution by the 42nd Amendment Act of the constitution
98. Find out the correct sequence in which the given terms are mentioned in the Preamble of the Indian Constitution :
(A) Sovereign, Democratic, Secular, Socialist, Republic
(B) Sovereign, Socialist, Secular, Democratic, Republic
(C) Secular, Sovereign, Democratic, Socialist, Republic
(D) Socialist, Secular, Sovereign, Democratic, Republic
99. Find out the extremists in the Indian National Movement :
(a) Bal Gangadhar Tilak
(b) Lala Lajpat Rai
(c) Bipin Chandra Pal
(d) Gopala Krishna Gokhale
(A) (a), (b) and (c)
(B) (a), (c) and (d)
(C) (a), (b) and (d)
(D) None
100. The Ministry of Urban Development under swachh Bharat Abhiyan scheme rated cleanestcities in India (Cities above 1 million population) in 2021. Write the sequential order (top tobottom) of the cleanest cities as per the survey conducted by the Central pollution control
board :
(A) Indore, Vijayawada, Surat, Navi Mumbai
(B) Surat, Vijayawada, Indore, Navi Mumbai
(C) Indore, Surat, Vijayawada, Navi Mumbai
(D) Navi Mumbai, Indore, Surat, Vijayawada



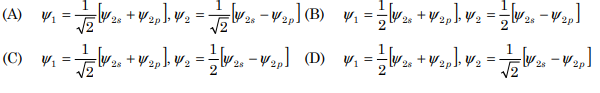





















No comments:
Post a Comment